Pediatric Oncology
The research program aims to (1) create new diagnostic and therapeutic options for children with cancer, (2) to develop new tumor classification systems based on innovative diagnostics and (3) to unravel mechanisms of tumorigenesis and to develop biomarker driven international combination early phase clinical trials.
Research Profile Dresden
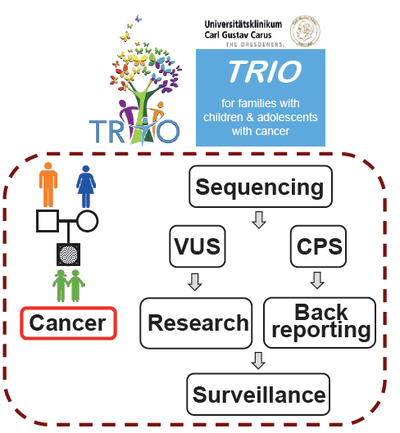
The Pediatric Oncology Program started its activity in 2019 at the medical campus Carl Gustav Carus TU Dresden. Understanding the genetic predisposition in childhood cancer with a focus on acute leukemia is intended and genetic predisposition is considered essential for disease evolution, therapy response and especially for toxicity and late effects. Thus, a clinical program (“TRIOstudy”) is set up offering exome sequencing of germline to all newly diagnosed children, including their parents at the pediatric oncology service at TU Dresden. In addition, the role of immune modulation and exposure to infection in children with acute leukemia is investigated, aiming for the prevention of the most common cancer of childhood.
TRIO study for children with cancer and their parents Germline predisposition contributes to approximately 10% of pediatric cancers. However, its actual contribution remains elusive. TRIO-Sequencing, which encompasses whole-exome sequencing of the affected child and its parents, presents a comprehensive approach to elucidate the etiology of the predisposition (inherited vs de novo). Since 2019, TRIO-DD at the department for Pediatric Oncology (NCT/UCC Dresden) was established, which offers TRIO-Sequencing to every child newly diagnosed with cancer and its parents, including a structured assessment on the clinical history and toxicity. In 2019, 78 children with cancer including 29% brain tumor, 28% leukemia/ lymphoma, 17% solid tumor, 11% sarcoma, 4% neuroblastoma and 11% other tumor patients were eligible with a study inclusion rate of approximately 80%. Sequencing and clinical data of the TRIO-DD cohort are being cross-validated with a TRIO-D cohort (DKTK Site Pediatric Oncology Düsseldorf ) encompassing 150 TRIOs of pediatric cancer cases. These data emphasize the urgent need for genetic testing in pediatric cancers independent of a positive family history or additional clinical findings.
Lin et al., Leukemia 2019; Martín-Lorenzo et al., Cancer Res 2018