NCT/UCC Immune Monitoring Unit
Specific immunotherapy is a promising treatment option for a number of different tumor patients. Strategies include vaccination with tumor peptides and immunostimulatory adjuvants, the administration of monoclonal and bispecific antibodies, and the adoptive transfer of non-modified and chimeric antigen receptor-engineered or T cell receptor-engineered T lymphocytes.
Monitoring the frequency, phenotype and functional properties of different immune cell populations is essential for understanding clinical responses and side effects caused by the treatment. Moreover, immune monitoring makes it possible to identify novel treatment-related prognostic and predictive biomarkers.
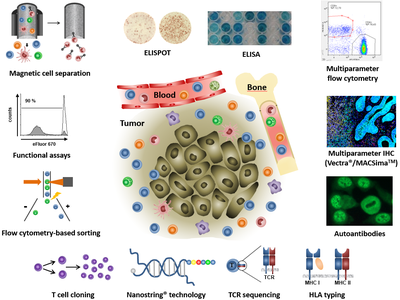
The major task of the Immune Monitoring Unit is to analyze the phenotype and functional properties of different immune cell populations in the context of clinical trials. Moreover, such studies support the design of improved immunotherapeutic strategies for tumor patients.
Services (selection)
- Monitoring different immune cell populations in the patient's blood during clinical studies
- Phenotypic and functional characterization of different immune cell populations in tumor samples and tumor-free tissues
- Consulting service for the design of immune monitoring for translational research projects and clinical studies
Methods
- Evaluation of tumor peptide-specific T cells (ELISPOT/Fow cytometry)
- Phenotyping of immune cells (FACS/ELISA/immunohistochemistry/immunofluorescence)
- Analysis of autoantibodies (immunohistochemistry/immunofluorescence)
- Functional assays for immune cells (Flow cytometry/ELISA/radioactivity assays)
- FACS-based single cell sorting
- Magnetic cell separation (MACS)
- T cell cloning
- T cell receptor sequencing
- Transcriptomics
- HLA typing
- Multiplex imaging platforms (Vectra®, PhenoImager HT®, MACSimaTM)
2025
Crespo E, Loureiro LR, Stammberger A, Hoffmann L, Berndt N, Hoffmann A, Dagostino C, Soto KEG, Rupp L, Arndt C, Schneider M, Ball CR, Bachmann M, Schmitz M, Feldmann A. RevCAR-mediated T cell response against PD-L1-expressing cells turns suppression into activation. NPJ Precis Oncol. 2025;9:42.
Rothe R, Golle T, Hachkar B, Hörz T, Pablik J, Rupp L, Dietsche I, Kruppa C, Fitze G, Schmitz M, Haase M, Wehner R. Tertiary lymphoid structures are associated with progression-free survival of peripheral neuroblastic tumor patients. Cancers. 2025;17:1303.
Tever O, Mentrup T, Chinn IK, Ishikuma H, Fluhrer R, Schmitz M, Wehner R, Behrendt R, Chinen J, Schröder B. The DNase TREX1 is a substrate of the intramembrane protease SPP with implications for disease pathogenesis. Cell Mol Life Sci. 2025;82:107.
Priego N, de Pablos-Aragoneses A, Perea-García M, Pieri V, Hernández-Oliver C, Álvaro-Espinosa L, Rojas A, Sánchez O, Steindl A, Caleiras E, García F, García-Martín S, Graña-Castro O, García-Mulero S, Serrano D, Velasco-Beltrán P, Jiménez-Lasheras B, Egia-Mendikute L, Rupp L, Stammberger A, Meinhardt M, Chaachou A, Martínez-Saez E, Bertero L, Cassoni P, Mangherini L, Pellerino A, Rudà R, Soffietti R, Al-Shahrour F, Saftig P, Sanz-Pamplona R, Schmitz M, Crocker SJ, Calvo A, Palazón A, RENACER23, Valiente M. TIMP1 mediates astrocyte-dependent local immunosuppression in brain metastasis acting on infiltrating CD8+ T cells. Cancer Discov. 2025;15:179-201.
2024
Messmer JM, Thommek C, Piechutta M, Venkataramani V, Wehner R, Westphal D, Schubert M, Mayer CD, Effern M, Berghoff AS, Hinze D, Helfrich I, Schadendorf D, Wick W, Hölzel M, Karreman MA, Winkler, F. T lymphocyte recruitment to melanoma brain tumors depends on distinct venous vessels. Immunity. 2024;57:2688-2703.
Salek M, Förster JD, Becker JP, Meyer M, Charoentong P, Lyu Y, Lindner K, Lotsch C, Volkmar M, Momburg F, Poschke I, Fröhling F, Schmitz M, Offringa R, Platten M, Jäger D, Zörnig I, Riemer AB. optiPRM: A targeted immunopeptidomics LC-MS workflow with ultra-high sensitivity for the detection of mutation-derived tumor neoepitopes from limited input material. Mol Cell Proteomics. 2024;23:100825.
Hilbig K, Towers R, Schmitz M, Bornhäuser M, Lennig P, Zhang Y. Cyclosporin A-based PROTACs can deplete abundant cellular cyclophilin A without influencing T-cell proliferation and cytokine production. Molecules. 2024;29:2779.
Kirchner J, Plesca I, Rothe R, Resag A, Löck S, Benešová I, Rupp L, Linge A, Wehner R, Krause M, Schmitz M. Type I conventional dendritic cells and CD8+ T cells predict favorable clinical outcome of head and neck squamous cell carcinoma patients. Front Immunol. 2024;15:1414298.
Igbo BT, Jentsch C, Linge A, Plesca I, Kuzay Y, Löck S, Kumaravadivel MS, Doms S, Stolz-Kieslich L, Pollack D, Brückmann S, Tittlbach H, Weitz J, Aust D, Apolle R, Schmitz M, Troost EGC. Correlation of microscopic tumor extension with tumor microenvironment in esophageal cancer patients. Strahlenther Onkol. 2024;200:595-604.
Grimm MO, Schostak M, Grün CB, Loidl W, Pichler M, Zimmermann U, Schmitz-Dräger B, Steiner T, Roghmann F, Niegisch G, Bolenz C, Schmitz M, Baretton G, Leucht K, Schumacher U, Foller S, Zengerling F, Meran J. Nivolumab + ipilimumab as immunotherapeutic boost in metastatic urothelial carcinoma: A nonrandomized clinical trial. JAMA Oncol. 2024;10:755-764.
Winter S, Schneider M, Oelschlaegel U, Maggioni G, Riva E, Raddi MG, Bencini S, Peruzzi B, Choy D, Antunes Dos Reis R, Güse E, Lischer C, Vera J, Timms JA, Sompairac N, Sockel K, Poloni A, Tunger A, Della Porta MG, Santini V, Schmitz M, Platzbecker U, Kordasti S. Mutations in the splicing factor SF3B1 are linked to frequent emergence of HLA-DR low/neg monocytes in lower-risk myelodysplastic neoplasms. Leukemia. 2024;38:1127-1131.
Winter S, Götze KS, Hecker JS, Metzeler KH, Guezguez B, Woods K, Medyouf H, Schäffer A, Schmitz M, Wehner R, Glauche I, Roeder I, Rauner M, Hofbauer LC, Platzbecker U. Clonal hematopoiesis and its impact on the aging osteo-hematopoietic niche. Leukemia. 2024;38:936-946.
Rupp L, Dietsche I, Kiessler M, Sommer U, Muckenhuber A, Steiger K, Van Eijck CWF, Richter L, Istvanffy R, Jäger C, Friess H, Van Eijck CHJ, Demir IE, Mota Reyes C, Schmitz M. Neoadjuvant chemotherapy is associated with suppression of the B cell-centered landscape in pancreatic ductal adenocarcinoma. Front Immunol. 2024;15: 1378190.
Ibneeva L, Singh SP, Sinha A, Eski SE, Wehner R, Rupp L, Kovtun I, Pérez-Valencia JA, Gerbaulet A, Reinhardt S, Wobus M, von Bonin M, Sancho J, Lund F, Dahl A, Schmitz M, Bornhäuser M, Chavakis C, Wielockx B, Grinenko T. CD38 promotes hematopoietic stem cell dormancy. PLoS Biol. 2024;22:e3002517.
Towers R, Trombello L, Fusenig M, Tunger A, Baumann AL, Savoldelli R, Wehner R, Fasslrinner F, Arndt C, Dazzi F, Von Bonin M, Feldmann F, Bachmann MP, Wobus M, Schmitz M, Bornhäuser M. Bone marrow-derived mesenchymal stromal cells obstruct AML-targeting CD8+ clonal effector and CAR T cell function while promoting a senescence-associated phenotype. Cancer Immunol Immunother. 2024;73:8.
Dinter L, Karitzky PC, Schulz A, Wurm A, Mehnert MC, Sergon M, Tunger A, Lesche M, Wehner R, Müller A, Käubler T, Niessner H, Dahl A, Beissert S, Schmitz M, Meier F, Seliger B, Westphal D. BRAF and MEK inhibitor combinations induce potent molecular and immunological effects in NRAS-mutant melanoma cells: Insights into mode of action and resistance mechanisms. Int J Cancer. 2024;154:1057-1072.
2023
Loureiro LR, Hoffmann L, Neuber C, Rupp L, Arndt C, Kegler A, Kubeil M, Hagemeyer CE, Stephan H, Schmitz M, Feldmann A, Bachmann M. Immunotheranostic target modules for imaging and navigation of UniCAR T-cells to strike FAP-expressing cells and the tumor microenvironment. J Exp Clin Cancer Res. 2023;42:341.
Grimm MO, Esteban E, Barthélémy P, Schmidinger M, Busch J, Valderrama BP, Charnley N, Schmitz M, Schumacher U, Leucht K, Foller S, Baretton G, Duran I, de Velasco G, Priou F, Maroto P, Albiges L. Tailored Approach with nivolumab and nivolumab/ipilimumab as immunotherapeutic boost in metastatic renal cell carcinoma. Lancet Oncol. 2023;24:1252-1265.
Nelde A, Schuster H, Heitmann JS, Bauer J, Mariner Y, Zwick M, Volkmer JP, Chen JY, Paczulla Stanger AM, Lehmann A, Appiah B, Märklin M, Rücker-Braun E, Salih HR, Roerden M, Schroeder SM, Häring MF, Schlosser A, Schetelig J, Schmitz M, Boerries M, Köhler N, Lengerke C, Majeti R, Weissmann IL, Rammensee HG, Walz JS. Immune surveillance of acute myeloid leukemia is mediated by HLA-presented antigens on leukemia progenitor cells. Blood Cancer Discov. 2023;4:468-489
Bayerl F, Bejarano DA, Bertacchi G, Doffin AC, Gobbini E, Hubert M, Li L, Meiser P, Pedde AM, Posch W, Rupp L, Schlitzer A, Schmitz M, Schraml B, Uderhardt S, Valladeau-Guilemond J, Wilflingseder D, Zaderer V, Böttcher JP. Guidelines for visualization and analysis of DC in tissues using multiparameter fluorescence microscopy imaging methods. Eur J Immunol. 2023;51:e2249923
Rupp L, Resag A, Potkrajcic V, Warm V, Wehner R, Jöhrens K, Bösmüller H, Eckert F, Schmitz M. Prognostic impact of the post-teatment T cell composition and spatial organization in soft tissue sarcoma patients treated with neoadjuvant hyperthermic radio(chemo)therapy. Front Immunol. 2023;14:1185197.
Digomann D, Strack J, Heiduk M, Plesca I, Rupp L, Reiche C, Nikolaus S, Beer C, Sommer U, Schmitz M, Distler M, Weitz J, Seifert AM, Seifert L. VISTA ligation reduces anti-tumor T cell activity in pancreatic cancer. Cancers. 2023;15:2326.
Grimm MO, Grün CB, Niegisch G, Pichler M, Roghmann F, Schmitz-Dräger B, Baretton G, Schmitz M, Bolenz C, Foller S, Leucht K, Schumacher U, Schostak M, Meran J, Loidl W, Zengerling F. Tailored immunotherapy approach with nivolumab with or without ipilimumab in patients with advanced transitional cell carcinoma after platinum-based chemotherapy (TITAN-TCC): a multicentre, single arm, phase 2 trial. Lancet Oncol. 2023;24:347-359.
2022
Plesca I, Müller L, Böttcher JP, Medyouf H, Wehner R, Schmitz M. Tumor-associated human dendritic cell subsets: phenotype, functional orientation, and clinical relevance. Eur J Immunol. In press.
Grimm, MO, Schmitz-Dräger BJ, Zimmermann U, Grün CB, Baretton GB, Schmitz M, Foller S, Leucht K, Schostak M, Zengerling F, Schumacher U, Loidl W, Meran J. Tailored Immunotherapy Approach with Nivolumab in advanced transitional cell carcinoma (TITAN-TCC). J Clin Oncol. 2022;40:2128-2137.
Peuker K, Strigli A, Tauriello DVF, Hendricks A, von Schönfels W, Burmeister G, Brosch M, Herrmann A, Krüger S, Nitsche J, Južnić L, Geissler MM, Hiergeist A, Gessner A, Wirbel J, Priyadarshini Ponnudurai R, Voogdt CGP, Tunger A, Wehner R, Stange DE, Weitz J, Aust DE, Baretton GB, Schmitz M, Röcken C, Hampe J, Hinz S, Zeller G, Chavakis T, Schafmayer C, Batlle E, Zeissig S. Integration of microbial signals by myeloid calcineurin controls anti-tumor immunity in colorectal cancer. Immunity. 2022;55:701-717.
Mentrup T, Stumpff-Niggemann AY, Leinung N, Schlosser C, Schubert K, Wehner R, Tunger A, Schatz V, Neubert P, Gradtke AC, Wolf J, Rose-John S, Saftig P, Dalpke A, Jantsch J, Schmitz M, Fluhrer R, Jacobsen ID, Schröder B. Phagosomal signalling of the C-type lectin receptor Dectin-1 is terminated by intramembrane proteolysis. Nat Commun. 2022;13:1880
Plesca I, Benešová I, Beer C, Sommer U, Müller L, Wehner R, Heiduk M, Aust D, Baretton G, Bachmann MP, Feldmann A, Weitz J, Seifert L, Seifert AM, Schmitz M. Clinical significance of tumor-infiltrating conventional and plasmacytoid dendritic cells in pancreatic ductal adenocarcinoma. Cancers. 2022;14:1216.
2021
Augsberger C, Hänel G, Xu W, Pulko V, Hanisch LJ, Augustin A, Challier J, Hunt K, Vick B, Rovatti PE, Krupka C, Rothe M, Schönle A, Sam J, Lezan E, Ducret A, Ortiz-Franyuti D, Walz AC, Benz J, Bujotzek A, Lichtenegger FS, Gassner C, Carpy A, Lyamichev V, Patel J, Konstandin NP, Tunger A, Schmitz M, von Bergwelt-Baildon M, Spiekermann K, Vago L, Jeremias I, Marrer-Berger E, Umana P, Klein C, Subklewe M. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC specific T cell bispecific antibody. Blood. 2021;138:2655-2669.
Nathansen J, Meyer F, Müller L, Schmitz M, Borgmann K, Dubrovska A. Beyond The Double-strand Breaks: The Role of DNA Repair Proteins in Cancer Stem Cell Regulation. Cancers. 2021;13:4818.
Berndt N, Bippes CC, Michalk I, Bartsch T, Arndt C, Puentes-Cala E, Soto JA, Loureiro LR, Kegler A, Bachmann D, Gross JK, Gross T, Kurien BT, Scofield RH, Farris AD, James JA, Bergmann R, Schmitz M, Feldmann A, Bachmann MP. And yet it moves: Oxidation of the nuclear autoantigen La/SS-B is the driving force for nucleo-cytoplasmic shuttling. Int J Mol Sci. 2021;22:9699.
Branchi V, Esser L, Boden C, Jafari A, Henn J, Lingohr P, Gonzalez-Carmona M, Schmitz M, Weismüller TJ, Strassburg CP, Manekeller S, Kristiansen G, Kalff JC, Matthaei H, Toma MI. A combined TLR7/TLR9/GATA3 score can predict prognosis in biliary tract cancer. Diagnostics. 2021;11:1597.
Tirado-Gonzalez I, Descot A, Soetopo D, Nevmerzhitskaya A, Schäffer A, Kur IM, Czlonka E, Wachtel C, Tsoukala I, Müller L, Schäfer AL, Weitmann M, Dinse P, Alberto E, Buck MC, Landry JJM, Baying B, Slotta-Huspenina J, Roesler J, Harter PN, Kubasch AS, Meinel J, Elwakeel E, Strack E, Tran Quang C, Abdel-Wahab O, Schmitz M, Weigert A, Schmid T, Platzbecker U, Benes V, Ghysdael J, Bonig H, Götze KS, Rothlin CV, Ghosh S, Medhouf H. AXL inhibition in macrophages stimulates host-versus-leukemia immunity and eradicates naïve and treatment resistant leukemia. Cancer Discov. 2021;11:2924-2943.
Kießler M, Plesca I, Sommer U, Wehner R, Wilczkowski F, Müller L, Tunger A, Lai X, Rentsch A, Peuker K, Zeissig S, Seifert AM, Seifert L, Weitz J, Bachmann M, Bornhäuser M, Aust D, Baretton G, Schmitz M. Tumor-infiltrating plasmacytoid dendritic cells are associated with survival in human colon cancer. J Immunother Cancer. 2021;9:e001813.
Seifert L, Plesca I, Müller L, Sommer U, Heiduk M, von Renesse J, Digomann D, Glück J, Klimova A, Weitz J, Schmitz M, Seifert AM. LAG-3- expressing tumor-infiltrating T cells are associated with reduced disease-free survival in pancreatic cancer. Cancers. 2021;13:1297.
Berndt N, Bippes CC, Michalk I, Bachmann D, Bachmann J, Puentes-Cala E, Bartsch T, Loureiro LR, Kegler A, Bergmann R, Gross JK, Gross T, Kurien BT, Scofield RH, Farris AD, James JA, Schmitz M, Fahmy K, Feldmann A, Arndt C, Bachmann MP. Two be or not two be: The nuclear autoantigen La/SS-B is able to form dimmers and oligomers in a redox-dependent manner. Int J Mol Sci. 2021;22:3377.
Bachmann MP, Bartsch T, Bippes CC, Bachmann D, Puentes-Cala E, Bachmann J, Bartsch H, Arndt C, Koristka S, Loureiro LR, Kegler A, Laube M, Gross JK, Gross T, Kurien BT, Scofield RH, Farris AD, James JA, Schmitz M, Feldmann A. T cell mediated conversion of a non-anti-La reactive B cell to an autoreactive anti-La B cell by somatic hypermutation. Int J Mol Sci. 2021;22:1198.
Müller L, Tunger A, Wobus M, von Bonin M, Towers R, Bornhäuser M, Dazzi F, Wehner R, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells: An update. Front Cell Dev Biol. 2021;9:637725.
2020
Seifert AM, Eymer A, Heiduk M, Wehner R, Tunger A, von Renesse J, Decker R, Aust DE, Welsch T, Reissfelder C, Weitz J, Schmitz M, Seifert L. PD-1 expression by lymph node and intratumoral regulatory T cells is associated with lymph node metastasis in pancreatic cancer. Cancers. 2020;12:E2756.
Pearson¬ FE, Tullett KM, Leal-Rojas IM, Haigh OL, Masterman KA, Walpole C, Bridgeman JS, McLaren JE, Ladell K, Miners K, Llewellyn-Lacey S, Price DA, Tunger A, Schmitz M, Miles JJ, Lahoud MH, Radford KJ. Human CLEC9A antibodies deliver Wilms’ tumor 1 (WT1) antigen to CD141+ dendritic cells to activate naïve and memory WT1-specific CD8+ T cells. Clin Transl Immunology. 2020;9:e1141.
Vadakekolathu J, Minden MD, Hood T, Church SE, Reeder S, Altmann H, Sullivan AH, Viboch EJ, Patel T, Ibrahimova N, Warren SE, Arruda A, Liang Y, Smith TH, Foulds GA, Bailey MD, Gowen-MacDonald J, Muth J, Schmitz M, Cesano A, Pockley AG, Valk PJM, Löwenberg B, Bornhäuser M, Tasian SK, Rettig MP, Davidson-Moncada JK, DiPersio JF, Rutella S. Immune landscapes predict chemotherapy resistance and immunotherapy response in acute myeloid leukemia. Sci Transl Med. 2020;12:eaaz0463.
Plesca I, Tunger A, Müller L, Wehner R, Lai X, Grimm MO, Rutella S, Bachmann M, Schmitz M. Characteristics of tumor-infiltrating lymphocytes prior to and during immune checkpoint inhibitor therapy. Front Immunol. 2020;11:364.
Müller L, Tunger A, Plesca I, Wehner R, Temme A, Westphal D, Meier F, Bachmann M, Schmitz M. Bidirectional crosstalk between cancer stem cells and immune cell subsets. Front Immunol. 2020;11:140.
Mitwasi N, Feldmann A, Arndt C, Koristka S, Berndt N, Jureczek J, Loureiro LR, Bergmann R, Mathe D, Hegedüs N, Kovacs T, Zhang C, Oberoi P, Jäger E, Seliger B, Rössig C, Temme A, Eitler J, Tonn T, Schmitz M, Hassel JC, Jäger D, Wels WS, Bachmann M. “UniCAR”-modified off-the-shelf NK-92 cells for targeting to GD2-expressing tumour cells. Sci Rep. 2020;10:2141.
Krüger T, Middeke JM, Stölzel F, Mütherig A, List C, Brandt K, Heidrich K, Teipel R, Ordemann R, Schuler U, Oelschlägel U, Wermke W, Kräter M, Herbig M, Wehner R, Schmitz M, Bornhäuser M, von Bonin M. Reliable isolation of human mesenchymal stromal cells from bone marrow biopsies in patients after allogeneic hematopoietic cell transplantation. Cytotherapy. 2020;22:21-26.
Arndt C, Loureiro LR, Feldmann A, Jureczek J, Bergmann R, Mathe D, Hegedus N, Berndt N, Koristka S, Mitwasi N, Fasslrinner F, Lamprecht C, Kegler A, Hoffmann A, Bartsch T, Köseer AS, Egan G, Schmitz M, Horejsi V, Krause M, Dubrovska A, Bachmann M. UniCAR T cell immunotherapy enables efficient elimination of radioresistant cancer cells. Oncoimmunology. 2020;9:1743036.
Binnewerg B, Schubert M, Voronkina A, Muzychka L, Wysokowski M, Petrenko I, Djurovic M, Kovalchuk V, Tsurkan M, Martinovic R, Bechmann N, Fursov A, Ivanenko VN, Tabachnick KR, Smolii OB, Joseph Y, Giovine M, Bornstein SR, Stelling AL, Tunger A, Schmitz M, Taniya OS, Kovalev IS, Zyryanov GV, Guan K, Ehrlich H. Marine biomaterials: biometric and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater Sci Eng C Mater Biol Appl. 2020;109:110566.
Gellrich FF, Schmitz M, Beissert S, Meier F. Anti-PD-1 and novel combinations in the treatment of melanoma – An update. J Clin Med. 2020,9:223.
2019
Rammensee HG, Wiesmüller KH, Chandran PA, Zelba H, Rusch E, Gouttefangeas C, Kowalewski DJ, Di Marco M, Haen SP, Walz JS, Gloria YC, Bödder J, Schertel JM, Tunger A, Müller L, Kießler M, Wehner R, Schmitz M, Jakobi M, Schneiderhan-Marra N, Klein R, Laske K, Artzner K, Backert L, Schuster H, Schwenck J, Weber ANR, Pichler B, Kneilling M, la Fougère C, Forchhammer S, Metzler G, Bauer J, Weide B, Schippert W, Stevanović, Löffler MW. A new synthetic toll-like receptor 1/2 ligand is an efficient adjuvant for peptide vaccination in a human volunteer. J Immunother Cancer. 2019;7:307.
Tunger A, Sommer U, Wehner R, Kubasch AS, Grimm MO, Bachmann M, Platzbecker U, Bornhäuser M, Baretton G, Schmitz M. The evolving landscape of biomarkers for anti-PD-1 or anti PD-L1 therapy. J Clin Med. 2019,8:E1534.
Ahmad F, Döbel T, Schmitz M, Schäkel K. Current concepts of 6-sulfo LacNAc- expressing monocytes (slanMo). Front Immunol. 2019;10:948.
Kegler A, Koristka S, Bergmann R, Berndt N, Arndt C, Feldmann A, Hoffmann A, Bornhäuser M, Schmitz M, Bachmann MP. T cells engrafted with a UniCAR 28/z outperform UniCAR BB/z-transduced T cells in the face of regulatory T cell-mediated immunosuppression. Oncoimmunology. 2019;7:e1621676.
Adhikaree J, Franks HA, Televantos C, Vaghela P, Kaur AP, Walker D, Schmitz M, Jackson AM, Patel PM. Impaired circulating myeloid CD1c+ dendritic cell function in human glioblastoma is restored by p38 inhibition - implications for the next generation of DC vaccines. Oncoimmunology. 2019;8:1593803.
Wagner F, Hölig U, Wilczkowski F, Plesca I, Sommer U, Wehner R, Maximilian Kießler M, Jarosch A, Flecke K, Arsova M, Tunger A, Bogner, Reißfelder C, Weitz J, Schäkel K, Troost EGC, Krause M, Folprecht G, Bornhäuser M, Bachmann MP, Aust D, Baretton G, Schmitz M. Neoadjuvant radiochemotherapy significantly alters the phenotype of plasmacytoid dendritic cells and 6-sulfo LacNAc+ monocytes in rectal cancer. Front Immunol. 2019;10:602.
2018
Kuske M, Westphal D, Wehner R, Schmitz M, Beissert S, Praetorius C, Meier F. Immunomodulatory effects of BRAF and MEK inhibitors: implications for melanoma therapy. Pharmacol Res. 2018;136:151-159.
Baran W, Oehrl S, Ahmad F, Döbel T, Alt C, Buske-Kirschbaum A, Schmitz M, Schäkel K. Phenotype, function and mobilization of 6-sulfo LacNAc-expressing monocytes in atopic dermatitis. Front Immunol. 2018;9:1352.
Olaru F, Döbel T, Lonsdorf AS, Oehrl S, Maas M, Enk AH, Schmitz M, Gröne EF, Gröne HJ, Schäkel K. Intracapillary immune complexes recruit and activate slan-expressing CD16+ monocytes in human lupus nephritis. JCI Insight. 2018;3:e96492.
Albert S, Arndt C, Koristka S, Berndt N, Bergmann R, Feldmann A, Schmitz M, Pietzsch J, Steinbach J, Bachmann M. From mono- to bivalent: improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo. Oncotarget. 2018;9:25597-25616.
Kubasch AS, Wehner R, Bazzuri S, Tunger A, Stasik S, Garzarolli M, Meinel J, Baretton G, Friedegund M, Thiede C, Schmitz M, Platzbecker U. Clinical, molecular and immunological responses to pembrolizumab treatment for synchronous melanoma and acute myeloid leukemia. Blood Adv. 2018;2:1187-1190.
Matko S, Manderla J, Bonsack M, Schmitz M, Bornhauser M, Tonn T, Odendahl M. PRAME peptide-specific CD8+ T cells represent the predominant response against leukemia-associated antigens (LAAs) in healthy individuals. Eur J Immunol. 2018;48:1400-1411.
Tunger A, Kießler M, Wehner R, Temme A, Meyer F, Bachmann M, Schmitz M. Immune monitoring of cancer patients prior and during CTLA-4 or PD1/PD-L1 inhibitor treatment. Biomedicines. 2018;6:26.
2017
Heidenreich F, Rücker-Braun E, Walz JS, Eugster A, Kühn D, Dietz S, Nelde A, Tunger A, Wehner R, Link CS, Middeke JM, Stölzel F, Tonn T, Stevanovic S, Rammensee HG, Bonifacio E, Bachmann M, Zeis M, Ehninger G, Bornhäuser M, Schetelig J, Schmitz M. Mass spectrometry-based identification of a naturally presented receptor tyrosine kinase-like orphan receptor 1-derived epitope recognized by CD8+ cytotoxic T cells. Haematologica. 2017;102:e460-e464.
Oehrl S, Olaru F, Kunze A, Maas M, Pezer S, Schmitz M, Schäkel K. Controlling the pro-inflammatory function of 6-sulfo LacNAc (slan) dendritic cells with dimethylfumarate. J Dermatol Sci. 2017;87:278-284.
Matko S, Teichert M, Tunger A, Schmitz M, Bornhauser M, Tonn T, Odendahl M. Enumeration of WT1-specific CD8+ T cells for clinical application using an MHC streptamer-based no-wash single-platform flow cytometric assay. Cytometry A. 2017;91:1001-1008.
Albert S, Arndt C, Feldmann A, Bergmann R, Bachmann D, Koristka S, Ludwig F, Ziller-Walter P, Kegler A, Gärtner S, Schmitz M, Ehninger A, Cartellieri M, Ehninger G, Pietzsch HJ, Pietzsch J, Steinbach J, Bachmann M. A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform. Oncoimmunology. 2017;6:e1287246.
Feldmann A, Arndt C, Bergmann R, Loff S, Cartellieri M, Bachmann D, Aliperta R, Hetzenecker M, Ludwig F, Albert S, Ziller-Walter P, Kegler A, Koristka S, Gärtner S, Schmitz M, Ehninger A, Ehninger G, Pietzsch J, Steinbach J, Bachmann M. Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology UniCAR. Oncotarget. 2017;8:31368-31385.
Tunger A, Wehner R, von Bonin M, Kühn D, Heidenreich F, Matko S, Nauerth M, Rücker-Braun E, Dietz S, Link CS, Eugster A, Odendahl M, Busch DH, Tonn T, Bonifacio E, Germeroth L, Schetelig J, Bachmann MP, Bornhäuser M, Schmitz M. Generation of high-avidity, WT1-reactive CD8+ cytotoxic T cell clones with anti-leukemic activity by streptamer technology. Leuk Lymphoma. 2017;58:1246-1249.
2016
Rücker-Braun E, Schetelig J, Link CS, Schmiedgen M, Tunger A, Vizjak P, Teipel R, Wehner R, Kuehn D, Fuchs YF, Oelschlaegel U, Germeroth L, Schmitz M, Bornhäuser M, Schetelig J, Heidenreich F. Longitudinal analyses of leukemia-associated antigen-specific CD8+ T cells in patients after allogeneic stem cell transplantation. Exp Hematol. 2016;44:1024-1033.
Temme A, Schmitz M. Chimeric antigen receptor-engineered primary natural killer cells: a tool to improve adoptive tumor immunotherapy. Immunotherapy. 2016;8:983-986.
Head
Coordination
Institute of Immunology, Medical Faculty Carl Gustav Carus, TU Dresden
Phone: +49 (0)351 458-6501
Email: marc.schmitz(at)tu-dresden.de
Dr. Rebekka Wehner
Institute of Immunology, Medical Faculty Carl Gustav Carus, TU Dresden
Phone: +49 (0)351 458-6524
Email: rebekka.wehner(at)tu-dresden.de
Staff
Cathleen Lippitsch
NCT/UCC Dresden
Phone: +49 (0)351 458-16528
Email: cathleen.lippitsch(at)nct-dresden.de
Susanne Doms
NCT/UCC Dresden
Phone: +49 (0)351 458-16524
Email: susanne.doms(at)nct-dresden.de