How artificial intelligence is expected to support cancer medicine
How artificial intelligence is expected to support cancer medicine
Artificial intelligence (AI) is increasingly finding its way into our everyday lives, and even into cancer medicine. It is not intended to replace medical personnel, but it does have the potential to support decision-making and to improve diagnosis and treatment. At the National Center for Tumor Diseases in Dresden (NCT/UCC), researchers are working with clinical experts on new AI-based applications for cancer medicine. To mark World Cancer Day on February 4, the NCT/UCC is providing an insight into AI research at the site.
The National Center for Tumor Diseases Dresden (NCT/UCC) is a joint institution of the German Cancer Research Center (DKFZ), University Hospital Carl Gustav Carus Dresden, the Faculty of Medicine Carl Gustav Carus of TU Dresden and the Helmholtz-Zentrum Dresden-Rossendorf (HZDR).
From speech recognition on our phones to spam filters on our PCs, and sat navs that pick routes to avoid traffic jams, intelligent programs are rapidly gaining in importance in our everyday lives. In cancer medicine too, there are numerous potential applications for AI-based systems, including in cancer diagnosis, when choosing the best therapy for an individual, and in surgery. Artificial intelligence is based on the lightning-fast analysis of huge volumes of data by computer programs that use self-adapting algorithms to identify patterns and regularities in the data. These can be patterns in images – such as particular cells or anatomical structures – or relationships between different types of data, such as laboratory results and information on the course of treatment. The lessons learned are generalized and stored in the form of mathematical models. Once the computer program has been trained, it is capable of responding appropriately to similar new events. AI methods range in complexity from comparatively simple algorithms, for instance ones that combine the results of different decision trees, to artificial neural networks, which link numerous mathematical functions together in a similar way to neurons in the human brain. “Despite all the advances in AI research, the responsibility for medical decisions still lies with the attending physician, and will continue to do so in the future,” says Professor Martin Bornhäuser, a member of the managing directorate of the National Center for Tumor Diseases in Dresden (NCT/UCC) and Director of Medical Clinic I of University Hospital Carl Gustav Carus Dresden. “Artificial intelligence can only provide important decision aids for the clinicians.”
Faster and more accurate cancer diagnosis
For cancer diagnosis, researchers at the NCT/UCC, University Hospital Dresden and TU Dresden have, for the first time, developed a computer system that can accurately identify acute myeloid leukemia (AML) and a mutation of importance for therapy using AI. Whereas conventional analysis of a bone marrow smear under the microscope requires a high level of expertise and takes several hours, the new algorithm can uncover abnormal cells and accurately identify the disease in less than ten seconds. The plan is for Dresden to start offering this diagnostic method as a global service in the near future.
There are also promising approaches in the area of skin cancer diagnosis. University Hospital Dresden is one of eight university hospitals across Germany taking part in a project that aims to translate an AI-based assistance system for the identification of malignant melanoma into routine clinical practice. The aim is to improve a powerful algorithm, which was previously trained on over 12,000 images, under real-world clinical conditions – because pictures can be taken on different devices and the way examining instruments are handled can vary between physicians. In order to prepare the AI for these variations and site-specific conditions, 100 melanomas and 100 benign skin lesions are currently being photographed in Dresden using a smartphone and dermatoscope, and then analyzed by experts and subjected to tissue analyses. The data will be used to train the computer system. The Skin Classification Project (SCP2) is being led by the German Cancer Research Center (DKFZ).
A system that is close to market entry is used in the diagnosis of breast and stomach cancer and is being tested by asgen, a Dresden-based start-up, together with the Institute of Pathology at University Hospital Dresden. The system analyzes whether a gene called HER2 is particularly active, which is a decisive factor for a potential targeted antibody therapy. The intelligent program recognizes cell nuclei and DNA segments marked with fluorescent dyes and can produce results much faster and more accurately than a sample-based manual count. It was trained on images of over 10,000 cell nuclei that had been marked up by hand. At the moment, it is being tested on around 100 patients alongside the standard diagnosis method. The aim is for it to support the work of pathologists in the future.
Individualizing and optimizing cancer therapy
Not only can AI contribute to a faster, more reliable diagnosis on the basis of known characteristics, but it can also help find new characteristics – known as biomarkers – that are relevant for the diagnosis or course of a disease. As part of a large-scale study by the German Cancer Consortium (DKTK), eight sites across Germany, led by the Dresden site, have been working since 2012 to identify new biomarkers and check whether they provide indications of the aggressiveness of head and neck tumors. Using various AI methods, Dresden researchers have identified several genes and characteristics in CT images as potential biomarkers. These are now being checked, along with other promising candidates. “In the future, the new biomarkers could be used to adapt radiotherapy individually for small groups of patients,” explains Professor Mechthild Krause, a member of the managing directorate of the NCT/UCC and Director of the Department of Radiotherapy and Radiooncology at University Hospital Dresden. “For instance, a higher radiation dose could be used for patients with particularly aggressive tumors.”
When choosing the best therapy for the individual, physicians need to have an overview of a large volume of information. More and more precise diagnoses and therapy options are making the data situation increasingly complex. Dresden-based hematologists are therefore taking part in the development of KAIT, an AI-based digital platform that is designed to provide and evaluate therapy-relevant data for various complex blood diseases. The system is fed with clinical guidelines, publications and studies and the results of several thousand structured clinical cases from registry data. The idea is that, in the future, an intelligent algorithm will filter and weight important information on the basis of individual patient data entered on the platform by the attending physician. Scientists in Dresden are contributing to the development of the system by selecting sources that are relevant for the clinical picture of acute myeloid leukemia (AML) and adapting the logarithm to weight the information specifically for this clinical picture. The collaborative project is supported by the Janssen company, and the research is being led by University Hospital Leipzig.
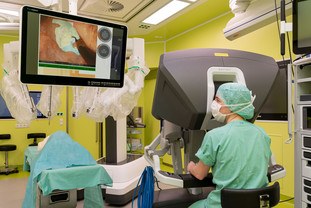
AI can also provide support during cancer treatment – for instance in the operating theater. The idea is for intelligent assistance systems to calculate the precise location of the tumor, for instance, and guide the surgeon during the intervention. Developing these kinds of systems is particularly difficult for interventions on soft tissue, which is constantly in motion, even during the operation. Here, unlike in orthopedics, neurosurgery or ENT medicine, computed tomography or magnetic resonance images produced before the operation do not provide reliable guidance. For robot-assisted rectal surgery, researchers at the NCT/UCC and at the Else Kröner Fresenius Center for Digital Health are therefore developing a system that recognizes important structures on the camera images from the patient’s abdomen and displays them in real time. On more than 25,000 individual images from operations, experts have drawn in by hand the optimum incision line and the nerves that need to be protected. Based on these, the software learned to recognize different stages of the operation, which can last for up to eight hours, and to display relevant information for the surgeon. “These aids are particularly relevant for rectal cancer surgery because just a few millimeters can mean the difference between sparing or losing nerves that are important for continence and sexual function,” explains Professor Jürgen Weitz, a member of the managing directorate of the NCT/UCC and Director of the Department for Visceral, Thoracic and Vascular Surgery at University Hospital Dresden. Over the course of this year, the new system will be tested during real-world operations.
Serious complications can occur during and after tumor operations in the abdomen. In the SurgOmics project, researchers from Dresden and Heidelberg are therefore developing an AI method designed to predict life-threatening complications. “The system is being trained on a large number of preoperative computed tomography images, information on patients’ pre-existing conditions, op videos from the abdomen and information about any complications that occurred. The aim is that, in future, the software will be available as an app that will warn physicians and nursing staff in real time in all treatment phases if complications are to be expected,” explains Professor Stefanie Speidel, Head of the NCT/UCC Department of Translational Surgical Oncology.
Many AI-based systems are so complex that it is almost impossible for users to see how the results are reached. But in medicine, in particular, transparency is extremely important to create trust and to be able to spot potential errors. Consequently, researchers from the Center for Advanced Systems Understanding (CASUS) at the Helmholtz-Zentrum Dresden-Rossendorf are working on explainable AI methods as part of OPTIMA, a European research program that collates clinical data from more than 200 million people with prostate, breast and lung cancer in a platform that complies with data protection standards. In a research area co-headed by the Pfizer company, they are developing AI models that can help clinicians and patient representatives understand how the software uses large volumes of data to support clinical decisions.