Immunotherapy
Based on a deep understanding of tumor-host immune interactions and the mechanisms tumors use to evade immunity, both sites develop strategies to re-induce tumor immunity. This includes strategies to modulate the tumor environment, strategies to actively guide T cells to the tumor cell and strategies to enhance host immunity by vaccination, or by reinfusion of potent effectors such as autologous antigen-specific T cells, chimeric antigen receptor transduced (CAR) T cells, or T cell receptors (TCR) transduced T cells. Fully human, fully immunocompetent tumor models have been developed that help to test such strategies preclinically and to identify potential mechanisms of resistance.
Research Profile Dresden
The Dresden translational immunotherapy program has focused on the development of modular bispecific antibodies and switchable chimeric antigen receptor transduced T cells (CAR-T) and natural killer cells (CARNK). In addition, adoptive cellular therapies employing virus- and tumor-reactive T cells (CAR-T, TCR-transduced T, Tumor Infiltrating Lymphocytes (TILs)) have been established. In a team effort of academic partners within the NCT and the university campus as well as two spin-off companies, the aim is to evaluate the modular BITE and the UniCAR platform in first-in-human clinical trials to provide a cure to patients with unmet medical needs.
UniCAR platform for cancer immunotherapy
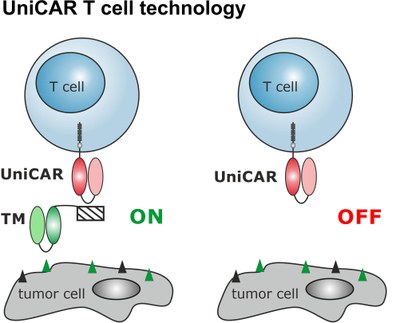
CAR T cells have clinically shown impressive results. However, due to the lack of any steering possibility, severe side effects can occur. To overcome this risk, a switchable CAR T cell platform was developed termed UniCAR. In contrast to conventional CARs, UniCAR T cells are switchable as they do not recognize the tumor target directly but require an adaptor molecule. As a consequence, UniCAR T cells are in an OFF mode in the absence of such a targeting module - but can be turned ON and OFF via infusion of the targeting module. As such, the activity can be regulated in a targeting module dose-dependent manner. So far, a series of targeting modules based on either antibody derivates or even small molecules against a variety of potential tumor targets were preclinically characterized and recently, a first clinical phase I trial was approved by the Paul Ehrlich Institute (NCT04230265).
Bachmann, Immunol Lett 2019; Arndt et al., Oncoimmunology 2019